
Prolistem for Non-Obstructive Azoospermia: A Comprehensive Guide
Prolistem for non obstructive azoospermia, Non-obstructive azoospermia (NOA)
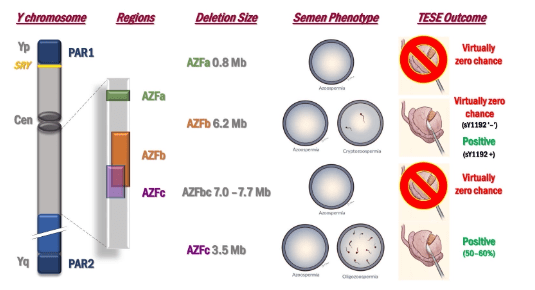
Genetic Causes of Azoospermia. Azoospermia can result from three primary etiological categories: hypothalamic-pituitary dysfunction, primary testicular failure leading to Non-Obstructive Azoospermia (NOA), and urogenital duct obstruction, termed Obstructive Azoospermia (OA). The condition is influenced by both acquired and congenital factors, with genetic contributions playing a significant role. Genetic testing, including karyotype analysis and screening for AZF microdeletions, is integral to diagnosing azoospermia, particularly NOA and OA.
The application of next-generation sequencing (NGS) has expanded the understanding of monogenic defects underlying NOA. Currently, 38 candidate genes are associated with various testicular histologies, including Sertoli Cell-Only Syndrome (SCOS), maturation arrest, and hypospermatogenesis. Key genes include:
Certain syndromes, such as Congenital Hypogonadotropic Hypogonadism (CHH) and Congenital Bilateral Absence of Vas Deferens (CBAVD), are linked to specific genetic mutations:
Advances in genetic understanding have improved diagnostic accuracy, patient counseling, and treatment strategies. Genetic testing guides the choice of interventions such as TESE or sperm cryopreservation. It also informs discussions on inheritance risks and the use of preimplantation genetic testing in assisted reproduction.
Future research aims to refine the genetic basis of idiopathic NOA, identify novel candidate genes, and integrate these findings into routine diagnostic workflows to enhance patient care.

Prolistem for non obstructive azoospermia, Non-obstructive azoospermia (NOA)

Introduction Male infertility, especially caused by azoospermia, affects

We are proud to have participated in the
Introduction: A New Hope in Male Infertility Treatment
Prolistem®, a patented male fertility formula for azoospermia and zero sperm count, has not been evaluated by the Food and Drug Administration. This product, designed to support sperm production restoration and male infertility treatment, is not intended to diagnose, treat, cure, or prevent any disease.
PROLISTEM® is a Patented Formula
Copyright © 2025 Prolistem®