
Male Infertility Symposium – Landmark Hotel, Amman
The Jordanian Society for Fertility and Genetics recently
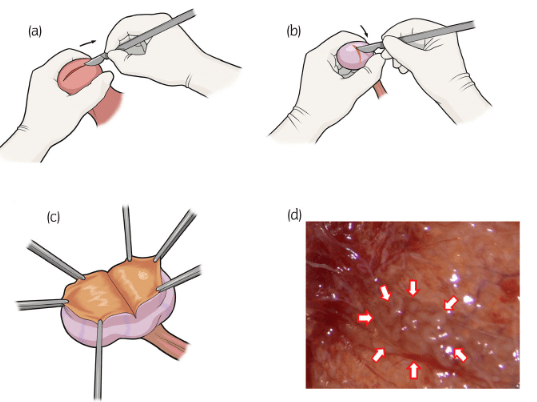
Nonobstructive azoospermia (NOA) is a condition marked by the absence of sperm in the ejaculate due to intrinsic testicular dysfunction. It affects approximately 1% of the general male population and up to 10% of infertile men. This document outlines the diagnostic and therapeutic approaches to managing NOA, including advancements and challenges in Non-obstructive Azoospermia Treatment.
Initial Evaluation:
Endocrinological and Genetic Tests:
Histological Evaluation:
Microdissection Testicular Sperm Extraction (Micro-TESE):
Varicocelectomy:
Gonadotropin Replacement Therapy:
Hormonal Therapy Pre-Treatment:
Repeated Micro-TESE:
Emerging Techniques:
Alternatives:
Biomarkers and Artificial Intelligence:
Regenerative Medicine:
NOA management has advanced significantly, with micro-TESE remaining the cornerstone of therapy. However, ongoing research into diagnostic biomarkers, predictive models, and regenerative treatments promises a future where even the most challenging cases of Non-obstructive Azoospermia Treatment can achieve successful outcomes.

The Jordanian Society for Fertility and Genetics recently

Prolistem for non obstructive azoospermia, Non-obstructive azoospermia (NOA)

Introduction Male infertility, especially caused by azoospermia, affects

We are proud to have participated in the